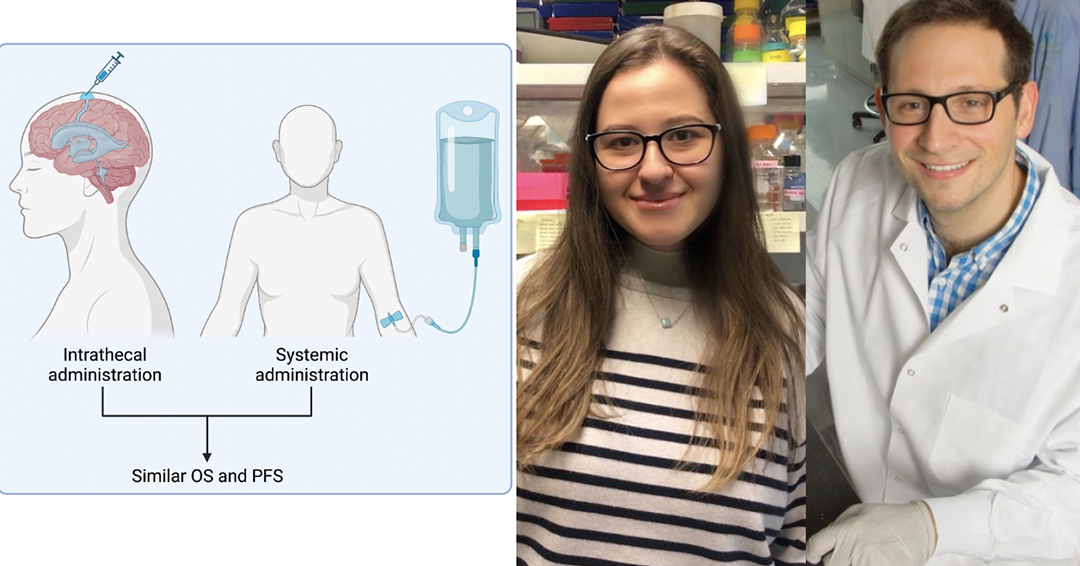
Anna-Maria Lazaratos, GCI trainee and MD/PhD student in the lab of Prof. Peter Siegel, studies cancer metastasis. She recently published as lead author in a paper investigating intrathecal trastuzumab versus alternate routes of delivery for HER2-targeted therapies in patients with HER2+ breast cancer leptomeningeal metastases.
Many centers around the world have been administering therapies intrathecally (directly into the cerebrospinal fluid of the brain) as standard of care for breast cancer that has metastasized to the leptomeninges, which are the outer layers of the brain and spinal cord. We wanted to determine whether there is any evidence to support that intrathecal administration is superior to administering the drug intravenously. We found that the outcomes with the 2 modalities are not significantly different from each other, sparing patients from needing to go through this difficult procedure to administer the drug intrathecally.
What do you think makes this paper stand out from other papers in the field?
Our paper is different from others in the field in that our study was the first to compare the efficacy of intrathecal versus oral and/or IV administration of HER2-targeted therapies in HER2+ breast cancer leptomeningeal metastasis.
What do you think is the most exciting finding from your paper and how do you think your lab, or other researchers, could expand on this work?
The exciting finding of the paper is the improved survival observed with trastuzumab deruxtecan, a new antibody-drug conjugate, compared to other therapies. These results have inspired our preclinical work, leading to the undertaking of a new project in Prof. Peter Siegel’s laboratory in collaboration with Matthew Dankner, a recent MD/PhD graduate, to elucidate the mechanism of trastuzumab deruxtecan in brain and leptomeningeal metastases. We hope that our results will lead to new trials with this drug for new subgroups of patients.
This project was inspired by and dedicated to my mom for her battle against leptomeningeal metastasis.
