An RI-MUHC team reveals a new mechanism by which the 100-year-old vaccine provides cross-protection against the influenza A virus
In a study published this week in the journal Nature Immunology, researchers from the Research Institute of the McGill University Health Centre (RI-MUHC) offer new insights into trained immunity, the ability of the innate immune system to form innate memory and provide long-lasting protection against infections.
For more than 100 years, the Bacillus Calmette Guérin (BCG) vaccine has been used to immunize children against Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB). Several studies have shown that BCG vaccination significantly reduces childhood mortality from other infectious diseases. However, the mechanisms of how BCG vaccination provides additional protection against unrelated pathogens remains unknown.
Maziar Divangahi, PhD, a professor of medicine at McGill University, pulmonary immunologist and senior scientist in the Translational Research in Respiratory Diseases Program at the RI‑MUHC, has been investigating the mechanisms of vaccine protections against infections.
“In this study, we aimed to mechanistically understand how BCG-vaccine, which is a live attenuated mycobacteria vaccine, can provide protection against viral pathogens,” says Prof. Divangahi. “We investigated the cellular and molecular mechanisms involved in BCG-mediated cross-protection against influenza A virus (IAV) infection.”
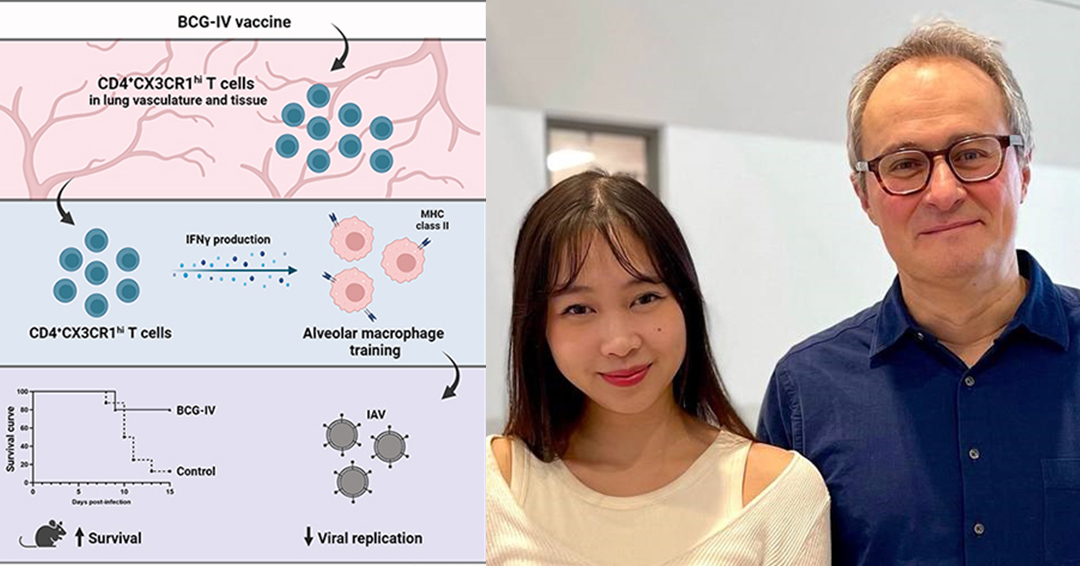
“We were very excited to discover that BCG vaccination provided remarkable protection against IAV infection,” explains first author Kim A. Tran, a PhD candidate in Prof. Divangahi’s lab. “The reduction of viral burden was dependent on interactions between memory T cells and one of the key innate residential cells in the lung, cells called alveolar macrophages. This is a previously unknown mechanism of cross-protection that is mediated by BCG. It highlights a crosstalk between the adaptive and innate immune memory system that is happening early on in infection.”
Conventionally, vaccine design involves the identification of a perfect antigen that generates long-term T and B cell memory responses and provides protective immunity when the same pathogen is next encountered. In this study, the researchers demonstrated that in response to the BCG vaccine, effector memory T cells for the mycobacteria expanded not only in the circulatory system, but also migrated into the lung tissue. Upon infection by the influenza virus, these BCG-specific memory T cells c can be activated and then “train” proximal innate immune cells (alveolar macrophages) in the lungs to limit influenza virus replication.
“The immune interactions involved here can ‘train’ the lungs, which are frequently exposed to infectious agents in the environment,” adds Prof. Divangahi. “If we can map out the protective immune pathways involved in the lungs, this will revolutionize our conceptual and clinical approaches in developing vaccines against infections, including emergent respiratory viruses.”
In past work, Prof Divangahi has highlighted the potent capacity of the BCG vaccine in inducing trained immunity, a novel concept in immunology whereby innate immune cells acquire memory-like properties. The current study provides the first evidence of how a vaccine can generate a dialogue between memory T cells and innate memory cells, resulting in cross-protection against an unrelated viral infection. The enrichment of effector memory T cells by BCG prior to IAV infection trains pulmonary innate cells to rapidly fight off the infection.
“These findings provide a basis for something that has been observed but not understood for decades – the protection provided by BCG against unrelated pathogens,” concludes Prof Divangahi. “Further work is needed to assess whether the BCG vaccine could eventually be used as a preventative tool against other emergent viral pathogens. It is exciting to note that concurrently, other groups have shown promising results when studying BCG protection against SARS-CoV-2, finding similar mechanisms to those identified in our study with the IAV.”
About the study
The authors gratefully acknowledge support from the CIHR (Canadian Institutes of Health Research), FRQS (Fonds de recherche du Québec) and the Richard and Edith Strauss Foundation.
The authors thank the Immunophenotyping Platform at the RI-MUHC.
Read the publication: Tran KA, Pernet E, Sadeghi M, Downey J, Chronopoulos J, Lapshina E, Tsai O, Kaufmann E, Ding J, Divangahi M. BCG immunization induces CX3CR1hi effector memory T cells to provide cross-protection via IFN-γ-mediated trained immunity. Nat Immunol. 2024 Jan 15. doi: 10.1038/s41590-023-01739-z. Epub ahead of print. PMID: 38225437
