The world’s first clinical trial comparing three alternative treatments for type 1 diabetes was conducted in Montréal by researchers at the IRCM and the University of Montreal, led by endocrinologist Dr. Rémi Rabasa-Lhoret, Adjunct Professor in the Department of Medicine (Division of Experimental Medicine) at McGill University. The study’s first author, Ahmad Haidar, is a postdoctoral fellow at McGill. The study confirms that the external artificial pancreas improves glucose control and reduces the risk of hypoglycemia compared to conventional diabetes treatment. The results, published today in the scientific journal The Lancet Diabetes & Endocrinology, could have a significant impact on the treatment of type 1 diabetes, a chronic disease that can cause vision loss and cardiovascular diseases.
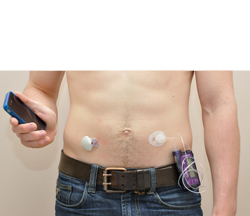
An emerging technology to treat type 1 diabetes, the external artificial pancreas is an automated system that simulates the normal pancreas by continuously adapting insulin delivery based on changes in glucose levels. Two configurations exist: the single-hormone artificial pancreas that delivers insulin alone and the dual-hormone artificial pancreas that delivers both insulin and glucagon. While insulin lowers blood glucose levels, glucagon has the opposite effect and raises glucose levels.
“Our clinical trial was the first to compare these two configurations of the artificial pancreas with the conventional diabetes treatment using an insulin pump,” says Dr. Rabasa-Lhoret, Director of the Obesity, Metabolism and Diabetes research clinic at the IRCM and professor at the University of Montreal’s Department of Nutrition. “We wanted to determine the usefulness of glucagon in the artificial pancreas, especially to prevent hypoglycemia, which remains the major barrier to reaching glycemic targets.”
Click here for the full press release
Related coverage
CTV | McGill doctor using insulin pump, phone app to measure blood sugar
