A new RI-MUHC research study identifies the role of neutrophil extracellular traps in promoting cancer metastasis to the lymph nodes
Published recently in the Journal of Extracellular Vesicles, a new study by researchers at the RI-MUHC may lead to better drugs to control the spread of cancer into the lymph nodes.
Led by Jonathan Cools-Lartigue, MD, PhD, a scientist in the Cancer Research Program at the Research Institute of the McGill University Health Centre (RI-MUHC), the team has revealed new mechanisms in lymph node metastasis, which could impact treatment of many early stage cancers.
Solid cancerous tumours are often treated in two ways, first by surgically removing the tumour, and next by attempting to prevent the tumour from spreading, or metastasizing, to other locations in the body. Most cancer deaths are caused by metastatic cancer, but the mechanisms of metastasis are complex and not well understood.
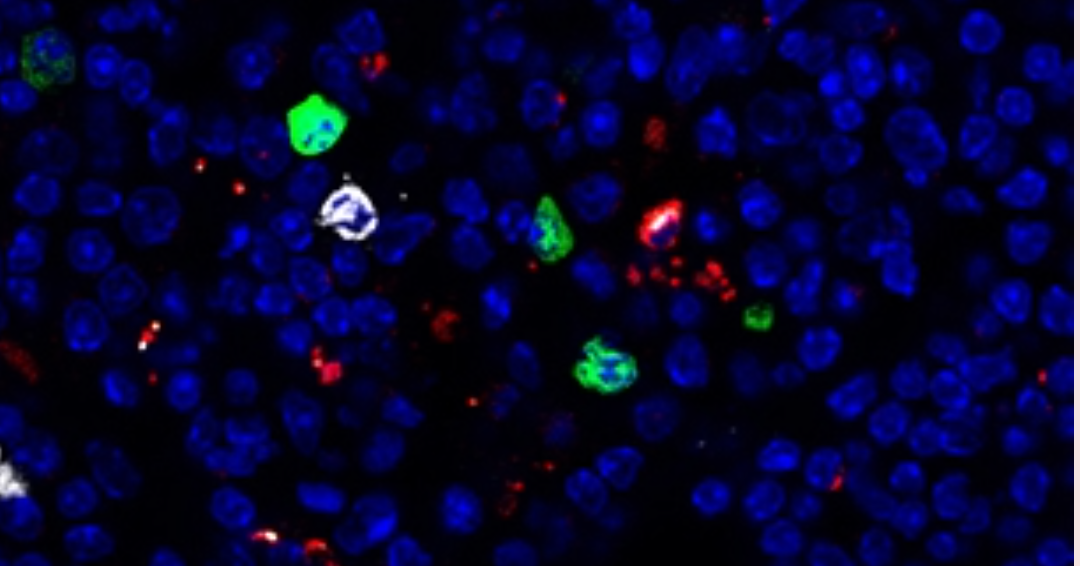
Lymph nodes are often the first place to which cancer will metastasize. In this new study, the research team were able to show that neutrophils, the most abundant white blood cells in the body, can actually promote metastasis in the lymph nodes.
“We know that neutrophils release web or mesh-like structures called neutrophil extracellular traps or NETs. With this study, we have learned that these NETs can prepare the lymph node in a way that helps cancer cells get established,” says Dr. Cools-Lartigue, who is also an assistant professor in the Department of Surgery of the Faculty of Medicine and Health Sciences at McGill University. “By learning more about how NETs work in the lymph nodes, we could then seek ways to inhibit their function in cancer metastasis.”
The researchers worked with tissue samples from 175 esophageal cancer patients, all of whom had been treated at the McGill University Health Centre. They found that high levels of NETs in these patients’ lymph nodes were tightly associated with decreased survival rates, indicating that the presence of NETs was enhancing cancer metastasis.
“To learn more about the mechanisms of NETs function in the lymph nodes, we built a model of cancer lymph node metastasis using a murine model. It was very challenging,” says Xin (Daniel) Su, MD, PhD, first author of the paper and a graduate trainee with Dr. Cools-Lartigue at the time of this work. “We selected 5 different cancer cell lines, labelled them using molecular tools, then linked them to the very small lymph nodes in the murine model.”
The researchers then identified and tested a variety of drugs to target NETs and neutrophils. By inhibiting NETs in this way, they found that they were able to successfully diminish lymph node metastasis in the model organism.
Finally, the team was able to demonstrate for the first time that extracellular vehicles (EVs) from the primary tumour were essential for the formation of NETs in lymph nodes. EVs are small particles produced by most cells in the body, including neutrophils. The researchers showed that, in response to EVs, endothelial cells in the lymph nodes secreted a protein called CXCL8/2, which in turn induced NETs formation and promoted metastasis in the lymph nodes.
The researchers anticipate that these results could inform the development of new treatments for cancer patients, such as more detailed pathological examination of lymph nodes in early stage cancer treatment and NETs based treatments to reduce lymph node metastasis.
About the study:
Read the publication: Tumour extracellular vesicles induce neutrophil extracellular traps to promote lymph node metastasis in the Journal of Extracellular Vesicles. First published: 10 August 2023 https://doi.org/10.1002/jev2.12341
The authors gratefully acknowledge funding from the Montreal General Hospital Foundation and The Thoracic Surgery Foundation. Dr. Xin Su received awards from MGH Foundation, MUHC Foundation and MUHC Department of Surgery.
The authors thank the Immunophenotyping Platform, the Molecular Imaging Platform, the Proteomics and Molecular Analysis Platform, and the Histopathology Platform at the RI‑MUHC, as well as the RI-MUHC biobank.
Related news
RI-MUHC study explores how to overcome the resistance of bladder tumours to radiation therapy
International consortium investigates overactive immune cells as cause of COVID-19 deaths
