In a paper recently published in Nature Partner Journals Precision Oncology, researchers from McGill’s Faculty of Medicine and Health Sciences provide evidence that suggests a particular group of immune-related genes could identify patients that might respond to immunotherapy across all cancer types.
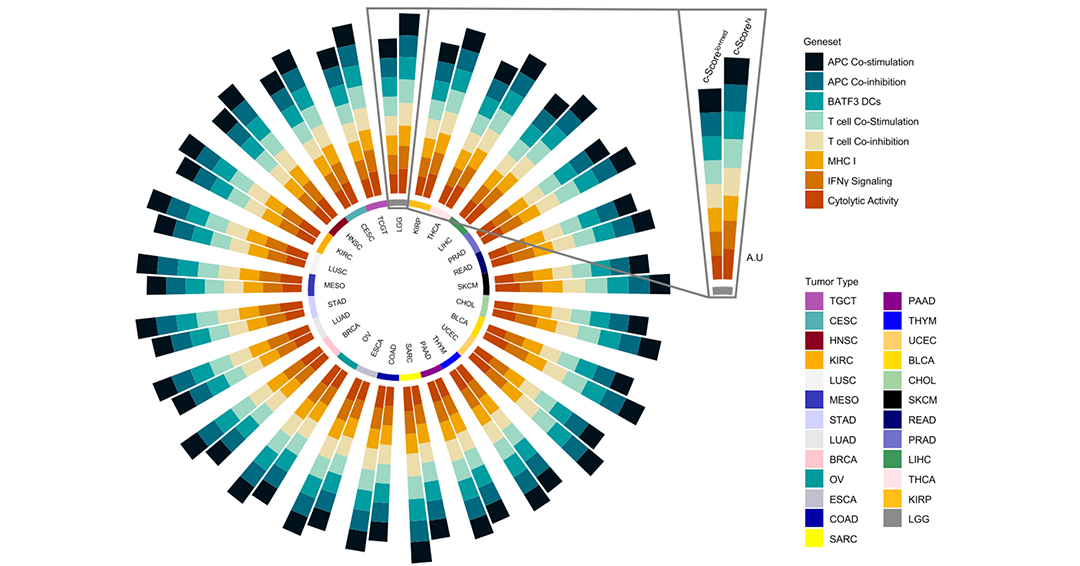
Titled, “Chemokine expression predicts T cell-inflammation and improved survival with checkpoint inhibition across solid cancers,” the paper, first-authored by MD-PhD student Joan Miguel Romero working under the supervision of surgeon-scientist George Zogopoulos, MD, PhD, Associate Professor in the Department of Surgery, reports that upon completing an investigation of different cancer types, subgroups of tumours displayed high expression of a chemokine score, or c-Score, with increased transcriptional hallmarks of the cancer-immunity cycle. “Across different cancer types, there are subpopulations that have high expression of this c-Score, and this is associated with a T cell-inflamed phenotype. This suggests these tumours could potentially respond to immunotherapy,” Romero explains.
Ideally, when an individual develops a tumour, if everything goes well, the body mounts an immune response, activating and releasing T cells, a special type of white blood cell. The T cells then go through the bloodstream searching for the tumour. The way that the body signals any type of immune cell to a specific location is by chemokines. The intended immune cell recognizes the specific chemokines and then it slowly starts moving towards this gradient of higher concentration until it reaches its target. Therefore, chemokines are responsible for recruiting immune cells.
“We wanted to see if chemokines could predict T cell infiltration into pancreatic cancer tumours. This is how we first identified this four-chemokine signature. A highly infiltrated tumour has a so-called ‘T cell-inflamed’ or ‘immune hot’ phenotype,’ which is needed for immune checkpoint inhibitors to work effectively. That’s why this signature is relevant in the context of the immunotherapy,” Romero notes.

One of the ways to determine likely candidates for immunotherapy is by conducting a biopsy, and then applying stains or dyes to view immune markers. Patients that have high expression of the gene signature could potentially be placed on immunotherapy, and current work is underway to further investigate the c-Score’s prognostic capability.
“The challenge is to identify patients that could respond to immunotherapy. We showed in the 2020 paper that there was a four-gene panel consisting of these chemokines that could identify tumours that displayed this T cell-inflamed phenotype,” Romero explains. “If you don’t have T-cells that are working, immunotherapy, like immune checkpoint blockade, won’t work in the tumour. Therefore, you need to have functional CD8+ T-cells that are present in the tumour, ready to go. We found that this signature could identify pancreas tumours, both primary and metastatic, that had this T cell-inflamed property or phenotype. For our recent paper, we wanted to further validate this signature by looking not just at pancreas cancer, but across different cancer histologies, including breast, ovarian, esophageal, rectal, lung, and others, and see if we could still identify patients that have this T cell-inflamed phenotype. We found that the c-Score was able to predict this T cell-inflamed property in a dataset of approximately 6,500 tumours. More importantly, now we wanted to also see whether this 4-chemokine signature was prognostic. Using three separate datasets spanning multiple studies across different cancers, we showed that the c-Score could predict response to immune checkpoint inhibition.”
While the current paper is promising, Romero notes that more work needs to be done and continues to focus on pancreas cancer, noting recent progress in that area. “An exciting part right now with pancreatic cancer research is identifying whether certain mutations make patients more likely to respond to immunotherapy.” One of these is the BRCA2 mutation. BRCA2, the same gene involved in breast cancer (along with BRCA1), provides instructions for making a protein that repairs DNA damage, thus acting as a tumour suppressor. Mutations in this gene can lead to homologous recombination deficiency (HRD), or a decreased ability to repair double strand breaks in DNA. “Studies have demonstrated improved survival for patients with BRCA2 mutations exhibiting HRD when given targeted chemotherapies. We and other groups are investigating whether giving these targeted therapies in combination with immunotherapies can help pancreas cancer patients harbouring BRCA2 mutations. This also highlights why genetic testing is so important in pancreas cancer. It helps identify patients with specific mutations that can then receive targeted therapies, marking a shift towards personalized oncology for this aggressive cancer,” Romero says.
Read the full paper here: doi: 10.1038/s41698-023-00428-2
